A Simple Apparatus for Determining the Relationship between Pressure and Temperature of Gases
Main Article Content
Abstract
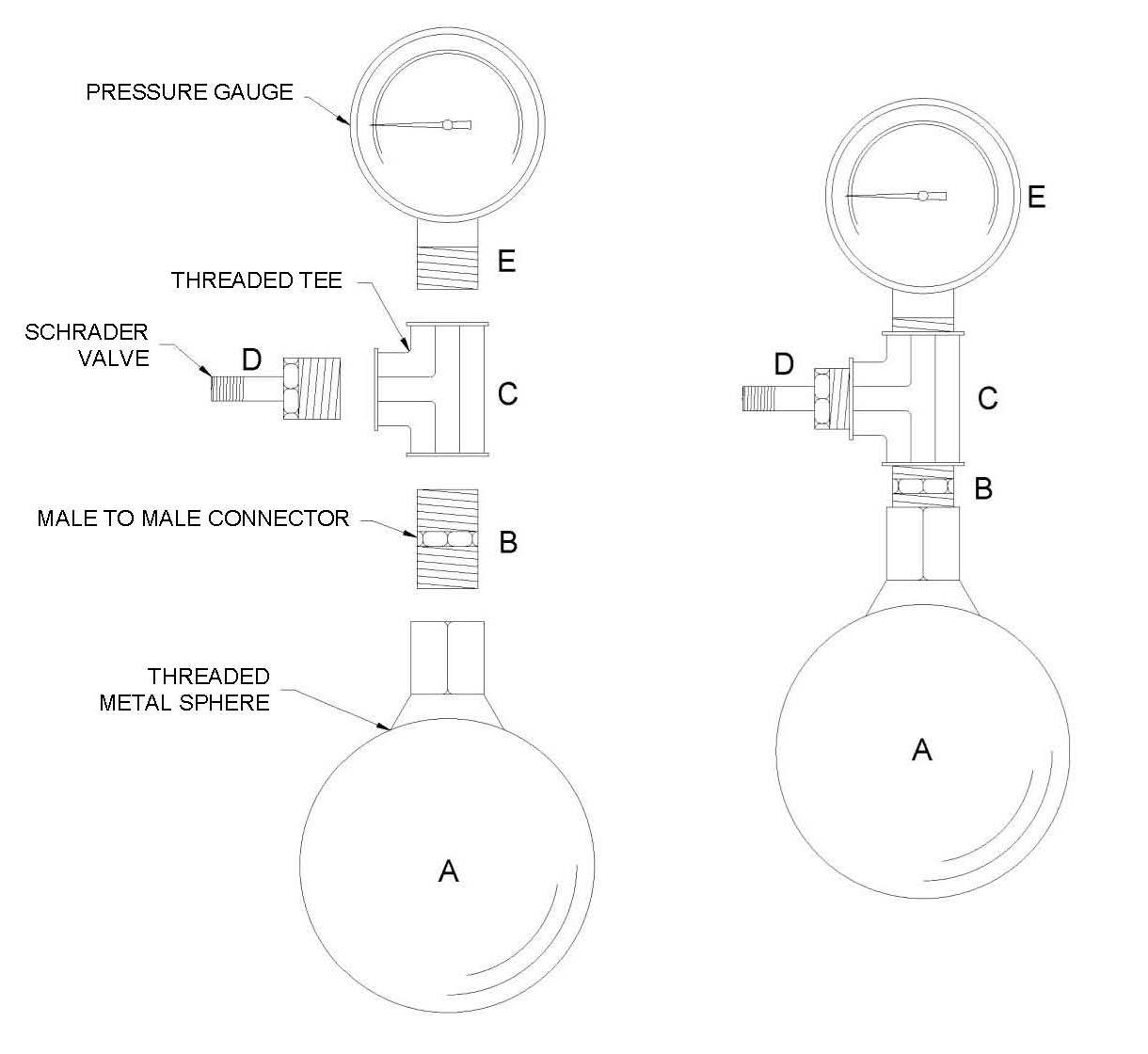
Nearly every high school and first-year college chemistry and physics course presents the topic of the gas laws. However, there are very few experiments effectively demonstrate the relationship between pressure and temperature of gases with a fixed volume, often referred to as the Gay-Lussac Law. This simple and cost effective apparatus is designed to allow students a hands-on experience when studying this concept. The apparatus consists of a pressurized stainless steel sphere connected to a pressure gauge. Testing of the apparatus determined that sphere sizes greater than 5.1 cm had significantly less error than smaller sizes. Sphere sizes between 6.4 and 11.4 cm had no statistical difference between them and had percent error values less than 4%. This apparatus could be an effective means of providing a hands-on exercise to demonstrate the Gay-Lussac Law in an introductory chemistry course.
Article Details
Copyright
Authors will be required to fill out the below copyright transfer form during the peer-review process and attach it along with their submission. In return, Knowledge Enterprises Journals grants authors the right to publish and reproduce the unrevised contribution in whole or in part at any time and in any form for any scholarly non-commercial purpose with the condition that all publications of the contribution include a full citation to the journal as published by Knowledge Enterprises Journals.
References
Dannelly, C.C. and M.E. Lash. 1950. “A lecture demonstration of Gay-Lussac’s Law.” J. Chem. Educ. 27(11), 618.
Randall, J. 2004. Advanced Chemistry with Vernier. Vernier Software and Technology, Beaverton, OR, USA.
Science First. 2009. Boyle’s Law and Absolute Zero. Yulee, FL, USA.
Zumdahl, S.S. and S.A. Zumdahl. 2014. Chemistry. 9th Ed. Cengage Learning, Belmont, CA, USA.